What is involved in digital health research?
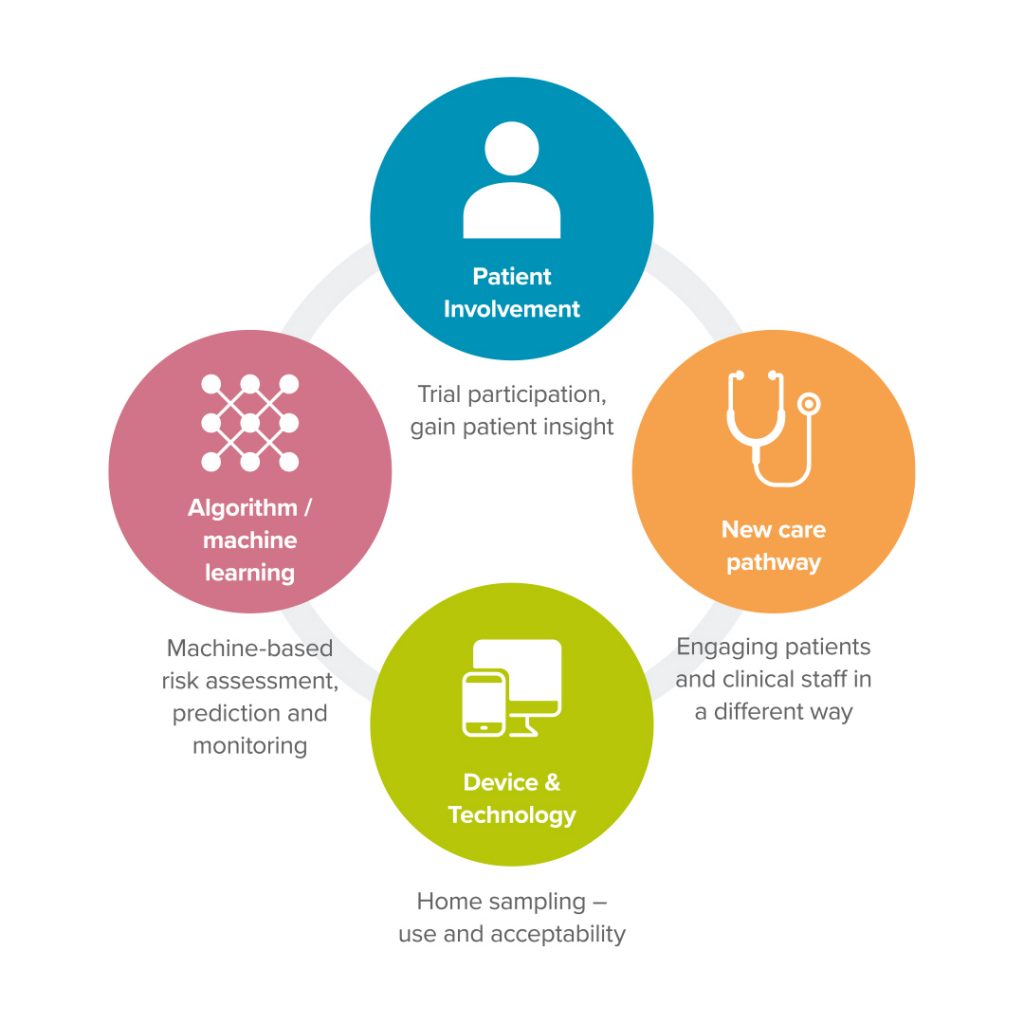
Advances in digital health and technology mean that we can conduct research into using technology and digital solutions for use in clinical trials to gather, interpret or analyse data. Our main research focus is designing and developing technology clinical trials. Depending on the clinical need this can involve several different areas of research – patient involvement, AI using machine learning, devices in the home and the development of new care pathways and digital biomarkers.
Our Research Areas
What is a technology clinical trial?
This type of clinical trial looks to test a technology or digital solution under the same clinical trial conditions as those used to test new drug treatments. This means that there needs to be a clear purpose and research question and an outcome measure that benefits patients.
Would you like to hear more?
Dr. Leanne Phillips né. Ogden describes the home-based monitoring of kidney function in the IN HOME trial
Making technology clinical trials a reality
We have a Design Lab within the clinical research facility at The Christie NHS Foundation Trust. Here our multi-disciplinary team can interact with patients and research nurses to get hands-on feedback about devices, insights into ideas, trial designs and how to take the technology clinical trials to the patient. Trials like:
In-Home
Phase 1 clinical studies often have eligibility criteria that prevent patients with reduced renal function from accessing the trial drug. Often these criteria are not reflective of the mechanism of the drug, or the toxicity profiles and these drugs remain untested in these populations until they are in mainstream circulation. Improved monitoring of renal function could potentially lead to earlier detection of adverse renal events and improve patient outcomes, alleviating the risk of patients with reduced renal function going on to clinical trials.
The IN-HOME study assesses the feasibility and acceptability of using a whole blood creatinine POCT device (NOVA Biomedical StatSensor® Xpress) to monitor patients kidney function whilst receiving potentially nephrotoxic anti-cancer treatments. digital ECMT have developed and implemented the end to end process by which creatinine can be monitored by patient in the home and an AKI assessment made and reported to an appropriate clinician. Part A of the trial opened in late in 2019, but due to COVID-19 had delays to recruitment when research trials were paused. In Q1 of 2021, Part A of the study completed successfully. Part B of the trial commenced in 2021, aiming to assess the clinical benefit of home-based creatinine monitoring by evaluating the potential for earlier diagnosis of AKI / change in renal function in cancer patients with intensive home monitoring.
NOTION
Immune related adverse events are side effects from cancer immunotherapies. These adverse events are very common with up to 90% of patients experiencing side effects on combination immunotherapies. Early evidence suggests that changes in cytokines correlate with the development of immune related adverse events. Therefore, monitoring of cytokines may help detect the onset of side effects and help manage them better for patients. The NOTION study (iN-hOme sampling of cyTokines in Immunotherapy patieNts) is a feasibility study to assess whether patients will take blood samples at home. The blood samples will be analysed retrospectively to assess whether changes in patients’ cytokines correlate to the development of immune-related adverse events.
A-EYE
The University of Manchester sponsored A-EYE study, aims to develop new AI methods to detect adverse retinal abnormalities associated with cancer treatment and assess against ophthalmologists’ decisions. This study opened to recruitment at the Manchester Royal Eye Hospital in June 2021. The data collected from the patients includes their anonymised eye scan alongside relevant clinical data. Our AI team are using these to develop and test an ethical and explainable algorithm to detect eye toxicities from eye scans.The University of Manchester sponsored A-EYE study, aims to develop new AI methods to detect adverse retinal abnormalities associated with cancer treatment and assess against ophthalmologists’ decisions. This study opened to recruitment at the Manchester Royal Eye Hospital in June 2021. The data collected from the patients includes their anonymised eye scan alongside relevant clinical data. Our AI team are using these to develop and test an ethical and explainable algorithm to detect eye toxicities from eye scans.
Got a digital health technology idea but not sure how to test it?
We are always on the look out for new ideas, and clinical research questions that we can apply to early cancer clinical trials. If you think we could help put those ideas into practice then lets start a conversation.